Coulomb’s Law Calculator
This Coulomb’s law calculator helps you calculate the attraction or repulsion between two static charged particles.
If the charges are equal, enter the Charge (q₁ & q₂), and distance (r) to calculate the electric force.
Enter charge 1 (q₁), charge 2 (q₂), and distance (r) to calculate the electric force if the charges are not equal.
Coulomb’s law states that attraction or repulsion between charged bodies is directly proportional to their product and inversely proportional to distance squared, acting along point charges.
You might be interested in Charles law or Boyles law.
What is Coulomb’s Law?
Coulomb’s law, named after French physicist Charles-Augustin de Coulomb, is a fundamental principle in electrostatics. It describes the force between two electrically charged particles. This force can be either attractive or repulsive, depending on the nature of the charges involved.
The law states that the magnitude of the electrostatic force between two point charges is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance between them. This relationship is similar to Newton’s law of universal gravitation, but it deals with electrical charges instead of masses.
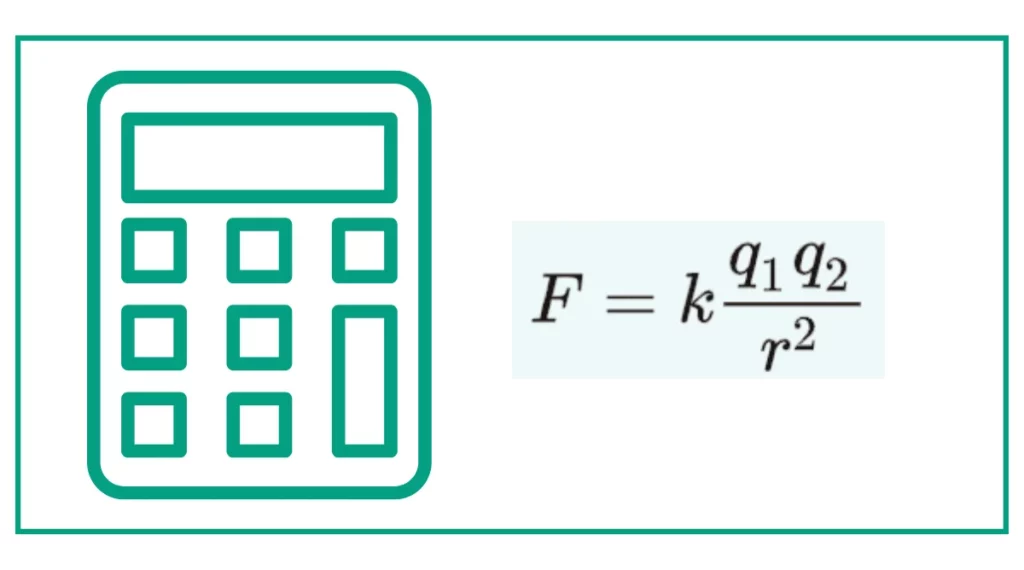
The Coulomb’s Law Formula
The mathematical expression of Coulomb’s law is:
F = k * (q1 * q2) / r^2
Where:
- F is the electrostatic force between the charges (measured in Newtons, N)
- k is Coulomb’s constant (approximately 8.99 × 10^9 N⋅m^2/C^2)
- q1 and q2 are the magnitudes of the charges (measured in Coulombs, C)
- r is the distance between the charges (measured in meters, m)
How to Use the Coulomb’s Law Calculator
Our online Coulomb’s Law Calculator is designed to make calculating electrostatic forces quick and easy. Here’s a step-by-step guide on how to use it:
- Choose the charge type: Select whether the charges are equal or not equal.
- Enter the charge values:
- If charges are equal, enter the value for both charges in nanocoulombs (nC).
- If charges are not equal, enter separate values for q1 and q2 in nanocoulombs (nC).
- Enter the distance between the charges in millimeters (mm).
- Click the “Calculate Force” button.
- The calculator will display the result, showing the electrostatic force in Newtons (N).
Examples of Coulomb’s Law Calculations
Let’s walk through two examples to illustrate how to use the Coulomb’s Law Calculator and interpret its results.
Example 1: Equal Charges
Suppose we have two equally charged particles, each with a charge of 5 nC, separated by a distance of 10 mm.
- Select “Charges are equal” from the dropdown menu.
- Enter 5 in the “Charge (q₁ & q₂)” field.
- Enter 10 in the “Distance (r)” field.
- Click “Calculate Force”.
The calculator will return a result of approximately 0.2246 N. This positive value indicates a repulsive force, as the charges have the same sign.
Example 2: Unequal Charges
Now, let’s consider two unequally charged particles: q1 = 3 nC and q2 = -7 nC, separated by a distance of 15 mm.
- Select “Charges are not equal” from the dropdown menu.
- Enter 3 in the “Charge 1 (q₁)” field and -7 in the “Charge 2 (q₂)” field.
- Enter 15 in the “Distance (r)” field.
- Click “Calculate Force”.
The calculator will return a result of approximately -0.0831 N. The negative value indicates an attractive force, as the charges have opposite signs.
Understanding the Results
When interpreting the results from the Coulomb’s Law Calculator, it’s important to remember a few key points:
- The magnitude of the force is given by the absolute value of the result.
- A positive result indicates a repulsive force (like charges).
- A negative result indicates an attractive force (opposite charges).
- The force acts along the line connecting the two charges.
Applications of Coulomb’s Law
Coulomb’s law has numerous applications in physics and engineering. Some of these include:
- Atomic structure: It helps explain the arrangement of electrons around the nucleus.
- Electrostatic precipitators: Used in industrial settings to remove particles from gas streams.
- Electrostatic spray painting: Utilizes the attraction between oppositely charged particles for efficient paint application.
- Van de Graaff generators: Devices that generate high voltages using principles of electrostatic charge accumulation.
Limitations of Coulomb’s Law
While Coulomb’s law is a powerful tool for understanding electrostatic interactions, it’s important to note its limitations:
- It assumes point charges, which may not always be accurate for real-world objects.
- It doesn’t account for the effects of other nearby charges or electric fields.
- At very small distances or with very large charges, quantum effects may become significant, and Coulomb’s law may not apply.
Solving Complex Physics Problems with Coulomb’s Law
The Coulomb’s Law Calculator is an excellent tool for solving simple two-charge problems. However, many real-world scenarios involve multiple charges or more complex configurations. In these cases, the principle of superposition is applied along with Coulomb’s law.
For a system of point charges, the net force on any one charge is the vector sum of the forces exerted by all other charges in the system. This principle allows us to extend Coulomb’s law to more complex scenarios.
The Relationship Between Coulomb’s Law and Electric Fields
Coulomb’s law is closely related to the concept of electric fields. An electric field is a region of space around a charged particle or object where an electric force would be experienced by another charged particle. The strength of this field at any point is defined as the force that would be experienced by a unit positive charge placed at that point.
The electric field strength (E) at a distance r from a point charge q is given by:
E = k * q / r^2
This formula is derived directly from Coulomb’s law and shows how the electric field strength decreases with the square of the distance from the charge.
Comparing Coulomb’s Law and Gravitational Force
Coulomb’s law bears a striking resemblance to Newton’s law of universal gravitation. Both forces follow an inverse-square relationship with distance. However, there are some key differences:
- Coulomb’s law deals with electric charges, while gravitational force acts between masses.
- Electrostatic forces can be both attractive and repulsive, while gravitational forces are always attractive.
- The electrostatic force is much stronger than the gravitational force for particles of similar size.
Advanced Applications: Coulomb’s Law in Nanotechnology
As we delve into the world of nanotechnology, Coulomb’s law becomes increasingly important. At the nanoscale, electrostatic forces often dominate over other forces like gravity. This principle is used in various applications:
- Atomic Force Microscopy (AFM): This technique uses the repulsive force between the atoms at the tip of a probe and the atoms of a sample surface to create high-resolution images.
- Nanoparticle assembly: Electrostatic interactions can be used to control the assembly of nanoparticles into larger structures.
- Drug delivery systems: Charged nanoparticles can be designed to interact with specific targets in the body based on their electrostatic properties.
References
- Jonson, J. O. (1997). The magnetic force between two currents explained using only Coulomb’s law. Chinese Journal of Physics, 35(2), 139-149.
- Jonson, J. O. (2008). Turning Back to Coulomb’s Law as a Basis for Electromagnetism. Proceedings of the NPA, 5(1), 113-118.